
Let us start by explaining that galvanic currents and stray currents are two different types of manifestations.
To understand what a galvanic current is, and consequently galvanic corrosion, we need to introduce some simple physics concepts that play a key role in this context:
Electrode: An electrode is a conductor of the first kind (e.g., metal or graphite) used to make electrical contact with a nonmetallic circuit part (e.g., a semiconductor, electrolyte, or vacuum). In an electrochemical solution, an electrode can be an anode or a cathode. Electrodes can be of 1st, 2nd, 3rd and 4th species.
Electrolyte: The term electrolyte generically refers to substances that undergo dissociation of their molecules into ions in solution. Substances that do not dissociate are called non-electrolytes. The term "electrolyte" is used to refer to the ability to conduct electric current due to the involvement of ions. Electrolytes, once dissolved in a solution, carry electric current due to the presence of two poles (negative and positive) within the solution. The transport is carried out by the ions themselves.
Ion: In chemistry, an "ion" is defined as an electrically charged molecular entity. In practice, when an atom (or a molecule or a group of atoms bound together) gives up or acquires one or more electrons it is transformed into an ion.
Galvanic Cell: A galvanic cell (or galvanic chain or voltaic cell) is a special electrochemical cell that allows chemical energy to be converted into electrical energy. A corrosion phenomenon or a battery is a classic example of a galvanic cell. The phenomenon of galvanic corrosion occurs when two different metals come into contact in the presence of an electrolyte (e.g., salt water) resulting in the creation of an unwanted natural galvanic cell that causes the involved metals to undergo the chemical corrosion reaction. In this case, the cathode and anode are represented by circumscribed areas of the metals involved, called the "microcathode" and "microanode."
Anode: The anode is the electrode on which a half-oxidation reaction takes place. In the case of a battery or galvanic cell, oxidation occurs spontaneously and produces electrons, so the anode is the negative pole. In the case of an electrolytic cell, oxidation is forced by subtracting electrons, so the anode is the positive pole. In electronic devices, the anode is the positive pole.
Cathode: In electrochemical systems, the càtode is the electrode on which a reduction half-reaction takes place. In the case of a battery or galvanic cell, reduction occurs spontaneously and consumes electrons, so the cathode is the positive pole. In the case of an electrolytic cell, reduction is forced by administering electrons, so the cathode is the negative pole. In electronic devices, the cathode is the negative pole.
Reduction: Reduction is the acquisition of one or more electrons by a chemical species. Each reduction occurs at the same time as an oxidation, which is the loss of electrons by another chemical species, so that electrons are exchanged by the two chemical species in question; the reactions of oxidation and reduction are thus two half-reactions that are part of that process of electron exchange, which is called oxidation-reduction.
Oxidation: Oxidation occurs when a chemical element undergoes electron subtraction, which results in an increase in its oxidation number. This subtraction of electrons can occur by another element, which thus undergoes the complementary process of reduction. Most oxidation reactions involve the development of energy in the form of heat, light or electricity. Substances that have the ability to oxidize other substances are known as oxidizing agents. They take electrons away from other substances, and because they basically accept electrons they are also called electron acceptors. Oxidizing agents are generally chemicals that possess high oxidation number elements, for example, hydrogen peroxide, permanganate or chromic anhydride, or highly electronegative substances, such as oxygen, fluorine, chlorine or bromine, capable of taking one or more electrons away from other substances.
Electrochemical behavior of materials: materials can be characterized by two types of behavior:
- active behavior, such as that of carbon steel, which, when it oxidizes, creates a spongy, compact layer on the surface (rust) that is unable to protect the underlying material;
- passive behavior, typical of stainless steel, which, when oxidizing, forms a thin compact layer capable of preventing the anodic process and thus protects the underlying material from corrosion.
WHAT THEY ARE
Now that we have introduced these basic concepts, let us turn to the two definitions of galvanic current and stray current:
Galvanic Current
A galvanic current is an electric current of weak intensity, which occurs due to the electric potential difference, which must still be high, between two different metals.
Due to galvanic current, the phenomenon that is called galvanic corrosion or electrolytic corrosion occurs. One of the most common types of phenomena is that created in the presence of moisture, where in the presence of water (in which the pH of the water plays an important role, in fact, water in the heating circuit if it has a low pH can generate corrosive phenomena) two metals with different nobility are in contact. In this case, electrons will migrate from the less noble metal to the more noble metal. The anode will be the less noble metal, which consequently will be the one that will oxidize. Conversely, the nobler metal will be the inert cathode, which in this particular case neither oxidizes nor reduces. The oxidizer is, of course, the oxygen present in the water.
Oxidation will be localized near the contact zone between the two metals, since the noble metal, as mentioned, does not undergo oxidation-reduction. Corrosion of metals will affect only the anodic part of the two metals, where the current is responsible for corrosion.
Corrosion of pipes, buried metal structures, or parts of a boat by galvanic marine currents, for example, is caused by the current that is created between the metals, causing small cracks, crevices, and holes.
These corrosions occur in the contact areas between metals, thus where direct joining occurs, welds, bolts, etc.Corrosions resulting from galvanic currents are quite dangerous, in that, they occur in the contact areas of metals, that is, in areas where direct joining occurs, in welds, bolts, brazing, etc.
The extent of corrosion is directly related to the following factors:
- By the difference in potential (hence nobility) of the two materials;
- by the amount of oxygen present;
- by the ratio of the total surface area of the two materials to that of the less noble metal (the smaller the less noble element the greater the extent of corrosion).
- From the salinity of the water; the saltier the water, the more conductivity there will be.
Roaming Current
Stray current, on the other hand, originates from a probable problem with the insulation or grounding of the electrical system.
The stray currents leave their normal path formed by the electrical conductors forming the circuit, to disperse and circulate outside the intended circuits and cross the ground where they encounter metal artifacts.
Keeping in mind that electric currents always seek paths where the least resistance is present, it always seeks to cross all those buried metallic elements.
If we are in the presence of leakage current, it will cross any metal structure placed in the ground, where the anodic part, that is, the area where the current exits, is subject to corrosion. As a result, they could cause corrosion.
This type of corrosion presents a higher intensity than galvanic or resulting from electrochemical reactions, which therefore causes very marked corrosion of metals, presenting localized degradation in the metal surface crossed by the current.
However, it is important to specify that stray current is harmful only if it is direct current and not the alternating current found in ordinary homes. In fact, the electric current in a house is never continuous except in rare cases. To better understand why we give a quick explanation of tupi of currents:
In an electrical system, electric current can be either direct or alternating. In the case of homes habitually, the alternating type is used because in this form one can easily switch from one voltage to another; in fact, only in specific cases and for certain needs is direct current used, which instead maintains its voltage constant.
More precisely, alternating voltage has an alternating pattern, this means that, for example, a voltage of 12V AC, has a pattern whose value alternates between +12V and -12V, that is, the voltage starts from 0V rises until it reaches its maximum positive +12V, then falls back to 0V, passes the latter heading toward negative values, reaches its maximum negative of -12V, and rises back to 0V, and then begins a new cycle again. This process is performed at an impressive speed that our eye cannot perceive. Normally in the 220V voltage that comes inside our homes, and thus in many apparatuses connected to it, this period is repeated 50 times per second, so it is said that the voltage has a frequency of 50 hertz.
The choice for alternating current arises from the high losses in direct current if the voltage is low, which, after all, is also true in alternating current. The convenience and efficiency of alternating current lies in the possibility of using transformers, which are devices to increase or decrease voltage depending on the application.
To this we must also add the fact that cement mortar offers high electrical resistance. Very often in cases of corrosion mistakenly attributed to stray currents the real problem is often caused by pipes carrying cold water and never those carrying gas heating. If stray currents were really the cause, one would have to think that stray currents are so "smart" as to select the pipe to be affected depending on its use.
HOW TO PREVENT THEM
There are several ways to protect metals from oxidation:
Adequate association of materials
It means to avoid associating galvanically incompatible materials with each other. The metals routinely used in plants are, from the least noble to the most noble:
- aluminum
- zinc
- steel
- lead
- brass
- copper
- bronze
- stainless steel
- titanium
- gold
- platinum
However, using materials that differ from each other does not automatically imply the presence of future galvanic corrosion. The discriminating factor is, in fact, the different electrical potentials. In fact, two metals that have similar or equal electrical potentials are called galvanically compatible metals, vice versa galvanically incompatible metals. The latter are those that can cause galvanic corrosion.
Keeping in mind that metals can exhibit active or passive behavior (see above), consequently, prevention can be passive or active:
Passive protection of plants by coating metals.
Galvanizing

Galvanizing is done by immersing a metal element in a zinc bath. In this way, zinc, being less noble than steel, acts as an anode and corrodes instead.
Zinc-treated iron remains intact until the entire zinc layer has been used up.
Insulation of the elements

Further protection from galvanic currents is provided by insulating elements at different potentials by applying protective coats of polyurethane, bitumen, polyethylene or other insulating materials.
This option, while valuable, involves a fairly substantial cost increase, and, in addition, could be subject to possible perforation or accidental wear that would render the insulation null and void.
In order to operate effectively, the coating of the insulation material must have a minimum length of 50 cm, otherwise it would lose effectiveness.
In the case where it is not possible to coat both metals, it will have to be the more noble metal to be coated, because if it were the less noble metal to be coated, in case the insulating layer deteriorates, we will have a very small anode area and a large cathode area that will result in high and rapid corrosion in the large area.
Dielectric joint

The dielectric joint is used in order to interrupt galvanic currents. The joint consists of two welded or threaded sockets, a protective layer on the outside and one on the inside.
Through the use of these simple dielectric joints, it is possible to make water and gas piping protected from dangerous galvanic corrosion, or to interpose an insulating layer, technically called a dielectric element, between the two materials, for example, a stainless steel piece joined to an aluminum piece by means of a pvc insulating layer.
By insulating the metals in this way, electrical passage between the metals and, consequently, corrosion is prevented.
Chrome plating

This is used as a protective coating of the surface of iron, where chromium among the noblest metals is electrolytically attached to iron.
Passivation of metals

Passivation is used to isolate the metal from external agents. In this process, anodic oxidation is carried out through the use of specific oxidizing products, making the metal resistant and adherent to the external surface.
Protective coating

This paint is applied at the points most prone to corrosion or along the entire length of the material.
Active plant protection
Impressed current cathodic protection
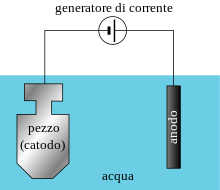
Cathodic protection is provided by the electromotive force applied by an external DC generator that generates a current, calibrated to balance the electromotive force causing the galvanic current.
The positive pole should be connected to an insoluble anodic earth electrode such as titanium, cast iron, graphite or silicon, while the negative pole should be connected to the metal to be protected.
Such a system, which uses very noble metals that do not wear out as easily, consumes as much electricity.
This type of galvanic protection finds application in concrete reinforcement, underground equipment, ship hulls, oil wells, marine or underground pipelines and for piers.
Sacrificial anode

The sacrificial anode is an additional element that is connected to the artefacts which corrodes in their place, protecting them. Obviously it must be an element composed of a less noble material than those it is intended to protect.
Altri articoli correlati:
Il calcestruzzo armato (cemento armato) - cos'è e le sue caratteristiche principali
"Umidità di risalita" cosa è e come contrastarla
"I principali tipi di pittura"
"Biocalce" rimedio innovativo contro l'umidità di risalita


Scrivi commento